Ross Maclean, MD | Executive Vice President, Partner - HEOR and Access Strategy
Liz Turner, MBA
The past few years have tested the resilience of even the boldest biotech innovators. From pandemic-era
trial delays to shifting venture capital priorities, the path to bringing life-changing treatments to patients with
rare diseases has grown increasingly complex. Meanwhile, rising expectations from regulators, payers, and
health technology assessment (HTA) bodies now demand more from every dollar spent, from broader population representation in trials to stronger proof of real-world impact.
Given these challenges, rare disease developers are even more focused on not just building, but communicating a compelling case for their therapies, while also showing their attention to financial impact. To do this, lean and targeted investments with smart, strategic decisions that demonstrate clinical value, economic impact, and long-term sustainability are critical.
This article concentrates on 5 high-impact areas where a focused investment can unlock value and build confidence with stakeholders.
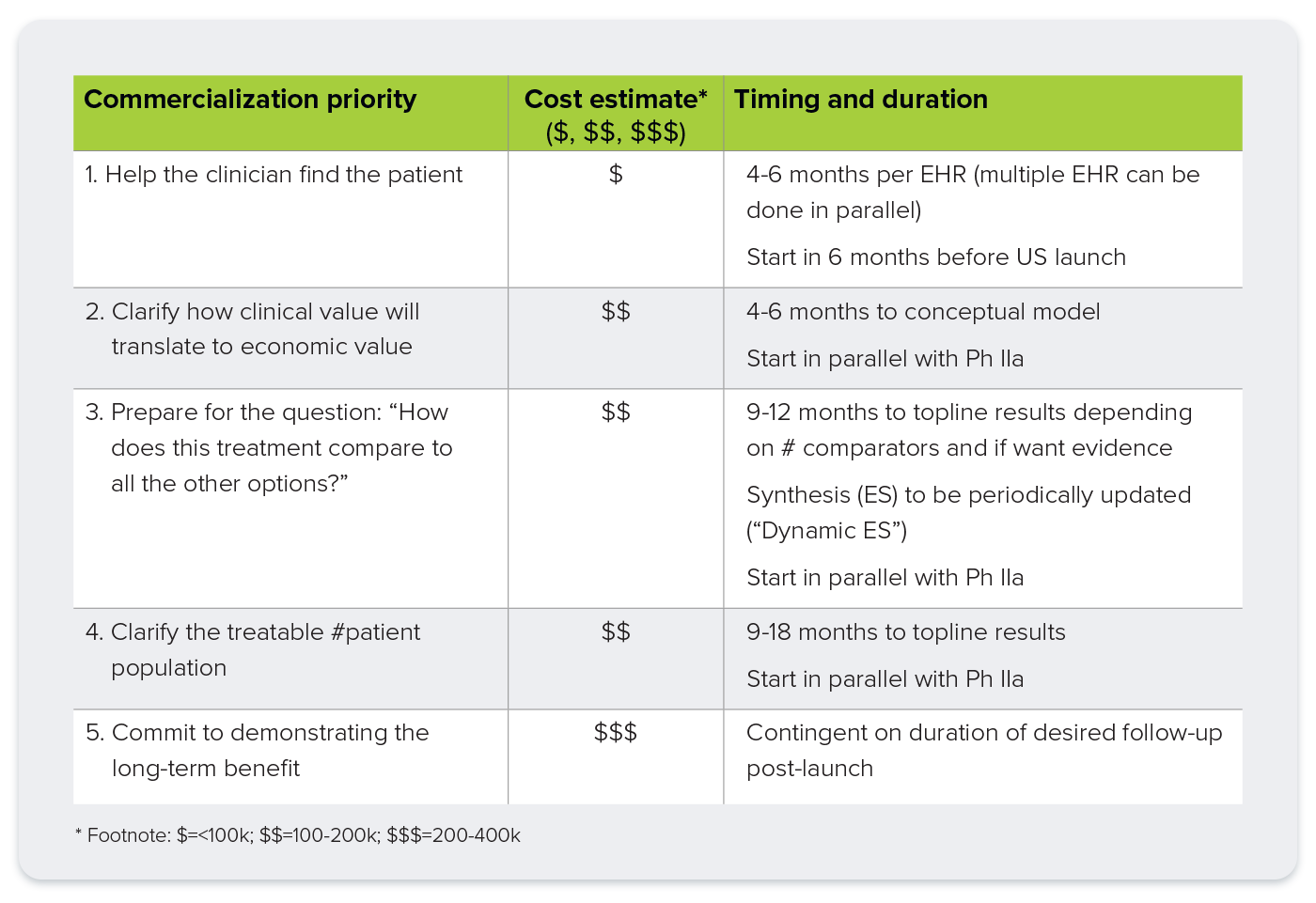
1. Clarify the Number of Patients “In-Play”
Why it matters: Payers are immediately concerned with affordability: how many patients will need this, and how much will it cost?
Precise patient identification is no longer optional. Tools such as genetic testing, biomarkers, and FDA-approved companion diagnostics give payers confidence in the size and scope of the treatable population. When insurers know exactly who the therapy is for, it lowers uncertainty and makes reimbursement conversations more straightforward. For ultra-rare diseases, where data can be sparse, a formal real-world data (RWD) landscape assessment may be needed to support these efforts and define unmet needs.
2. Help the Clinician Find the Patient
Why it matters: Earlier diagnosis improves outcomes and reduces long-term costs.
Rare diseases often present with non-specific symptoms and are difficult to diagnose. That’s where data-driven tools like electronic health record (EHR) “pursuit lists” come in, helping clinicians identify likely patients faster by scanning for telltale patterns. Advances in sequencing and AI-enhanced analytics make genetic testing more accessible and insightful. These tools not only increase diagnostic accuracy but also create opportunities for manufacturers to tailor treatments based on specific genetic profiles, while remembering this must be done with care to remain compliant with regulations around test interpretation.
3. Commit to Demonstrating Long-Term Benefit
Why it matters: Long-term value must be proven, not assumed.
Traditional approaches like long-term extensions, registries, and observational studies still have a role, but they can be expensive and time-consuming. A newer, more efficient option involves tokenization: assigning a unique, HIPAA compliant patient identifier to connect data across clinical trials and real-world datasets. This approach enables meaningful post-launch evidence that clinical trial success translates into real-world benefit. It also helps address equity by demonstrating impact across diverse populations, including rural or underserved groups.
4. Clarify How Clinical Value Translates to Economic Value
Why it matters: Payers and HTAs want to understand not just if a treatment works, but why it’s worth paying for.
An early clinical and economic model, built even before pivotal trial results, can identify key cost drivers, from
hospitalization rates to disease progression. This foresight allows innovators to tailor trial design and endpoints to the metrics that matter most to payers. These models provide early insight into potential reimbursement success, informs ICER strategy in the US, and forms the basis for cost-effectiveness models required in global HTA markets.
5. Prepare for the Question: “How Does This Compare?”
Why it matters: Comparative effectiveness is a must-have, not a nice-to-have.
Every stakeholder, from patients to regulators, wants to know how a new treatment stacks up against existing options. A structured evidence synthesis (ES), including network meta-analysis (NMA), gives you a head start on this inevitable question. ES can be refreshed as new data emerges and is essential for HTA submission outside the US Coupled with ongoing real-world evidence (RWE), it can help demonstrate that your therapy offers meaningful improvement over standard of care—justifying higher pricing where appropriate.
Summary: Smart Spending for Strategic Returns
Of course, these tactics raise the obvious questions related to cost and treatment duration. Implementing all 5 would require a significant investment. But for an R&D program likely to exceed tens or even hundreds of millions, this investment is a strategic safeguard. This targeted investment strategy could pay off substantially, providing the clear and credible answers that the C-suite, investors, and payers demand, ultimately improving the odds of future financing. This early and focused planning helps to deliver the evidence needed to support your desired outcome.
